From Discovery to CMC: Outsourcing via integrated partnerships simplifies the externalization journey
The advantages of a robust outsourcing strategy for pharma and biotech has become clear over the last decades. Outsourcing research and development that would have previously been carried out in-house allows capital efficiency by moving fixed costs into variable cost, and also capital elasticity, with the ability to adjust spending by portfolio needs and data. Outsourcing can also be used to address capacity shortfall, an internal expertise or capability gap and allows companies to focus on their core expertise while externalizing other activities.
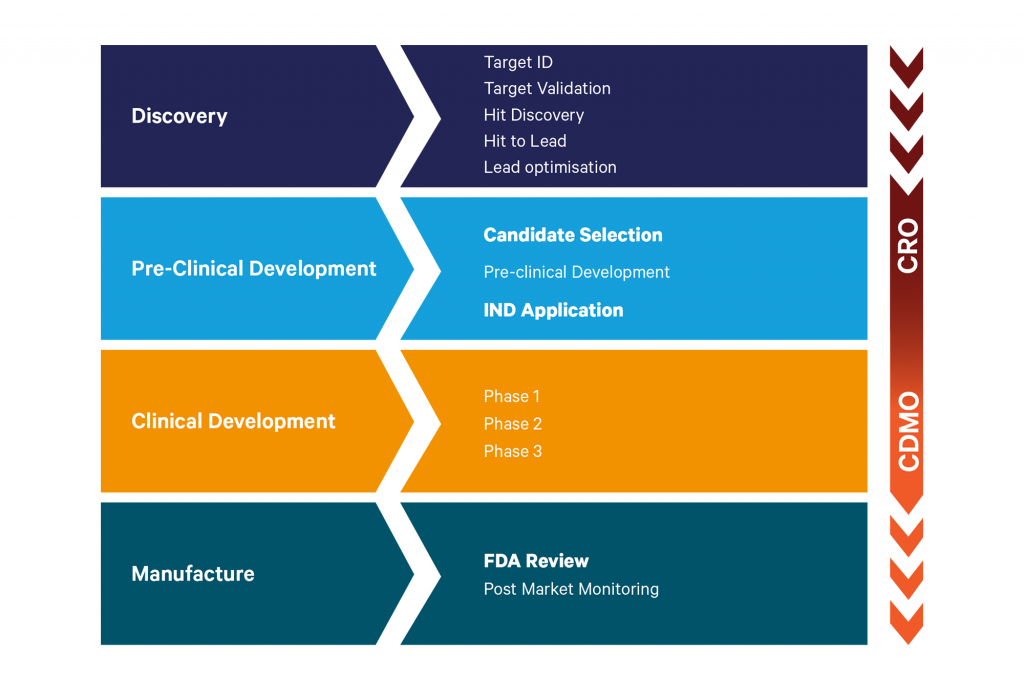
However, the outsourcing journey taken by pharma and biotech organisations to deliver a new small molecule drug onto the market can be highly complex, with multiple disciplines and external organisations engaged over many years.
Supporting companies with their outsourcing efforts are both contract research organisations (CROs) and contract development and manufacturing organisations (CDMOs). There is typically some overlap over where these organisations engage with customers and in the small molecule space, that happens typically as companies are approaching candidate selection, and are requiring scale up of non-GLP batches.
Outsourcing Strategies
Strategies towards outsourcing vary between organizations. Some companies prefer to use a wide variety of different suppliers, seeking the best specialists for the different components of the drug discovery process. Others prefer to work with providers of integrated drug discovery, where a multi-disciplinary project team’s drug discovery and development experience is brought to bear on a project from within a single CRO.
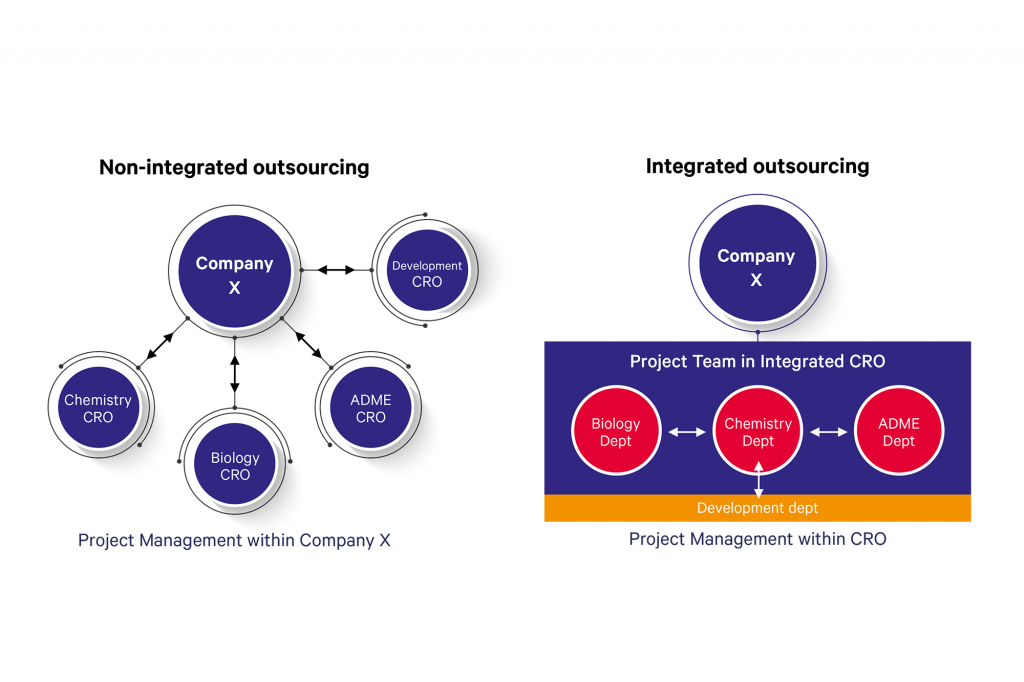
In a non-integrated scenario, the outsourcing company sub-contracts their work to different suppliers for different disciplines and carries out all the project management themselves. With an integrated outsourcing approach, a project team within the outsourcing company outsources a project to an integrated supplier, and a project team is set up within the CRO who coordinate the project at their co-located facilities with continual communication to the customer through regular project meetings. Project management, technology transfer and logistics are handled within the CRO.
There are many reasons that companies may choose to outsource integrated services. Minimising contractual and paperwork complexity, and simplifying project management and logistics can be advantageous when working with an integrated partner compared to engaging multiple CRO partners. Outsourcing companies may lack internal budget, resource or facilities or may wish to tap into external drug discovery and development experience as well as accessing specific experience, skills and capabilities.
Working with a ‘One Stop Shop’ enables the outsourcing company to access all these aspects from within a single CRO/CDMO organisation. Indeed, the rise of the virtual biotech, where companies do not have their own wet labs, is enabled by close relationships between the biotech and their integrated service providers. A good relationship between an outsourcing company and their solution provider can be a key reason to choose to work in an integrated way for both the drug discovery and development phases of a project’s life – the culture and values across an integrated CRO/CDMO that will be experienced by the outsourcing company are likely the same.
It is important to realise though, that even when CRO/CDMOs claim to be a one stop shop, they rarely have every capability that a particular project may require, and an honest, reliable CRO/CDMO will be open about their capability gaps. Some CRO/CDMOs may fill these gaps by sub-contracting to another provider while supplying the project management to the outsourcing company, while others will provide logistical support while the outsourcing company contracts directly with the non-integrated supplier.
One important decision when choosing an integrated CRO/CDMO partner is to have confidence in all disciplines within that organisation - an integrated supplier must be able to deliver on all aspects of a customer’s project with equal quality. Improving speed and efficiency can be a key aspect of working with an integrated provider, with co-located multidisciplinary facilities being optimum for logistics, team working and technology transfer. Certainly, when moving from the Discovery into Development phase, difficulties in technology transfer can be minimised by working within the same organisation and valuable inputs from the development team can be gained ahead of candidate selection.
So, with all these reasons to work with integrated suppliers, one may wonder why outsourcing companies sometimes choose not to. There can be many valid reasons not to work in an integrated fashion – requiring specialist services with specific expertise is a common reason. Wanting a range of suppliers for one discipline, but requiring standardised results from a single supplier in another discipline could be another valid reason. For example, 3 chemistry suppliers, but standardised ADME results across multiple programmes. Department heads may have their own preferred suppliers and companies may wish to mitigate risk by having supplier/geographic diversity of outsourcing activities. Legacy outsourcing strategies also play a part, as does having extensive internal outsourcing project management. Also, if a CRO/CDMO has a particular weakness in a certain discipline, then the outsourcing company may go elsewhere for that discipline. Finally, stretching the dollars may play a part, as the outsourcing company may be able to find services at lower cost at different organisations. The counter argument to this is that by placing work at an integrated solution provider, the outsourcing company may be able to leverage discounts dependent upon their overall spend with the supplier.
The changing face of outsourcing from a CRO/CDMO perspective
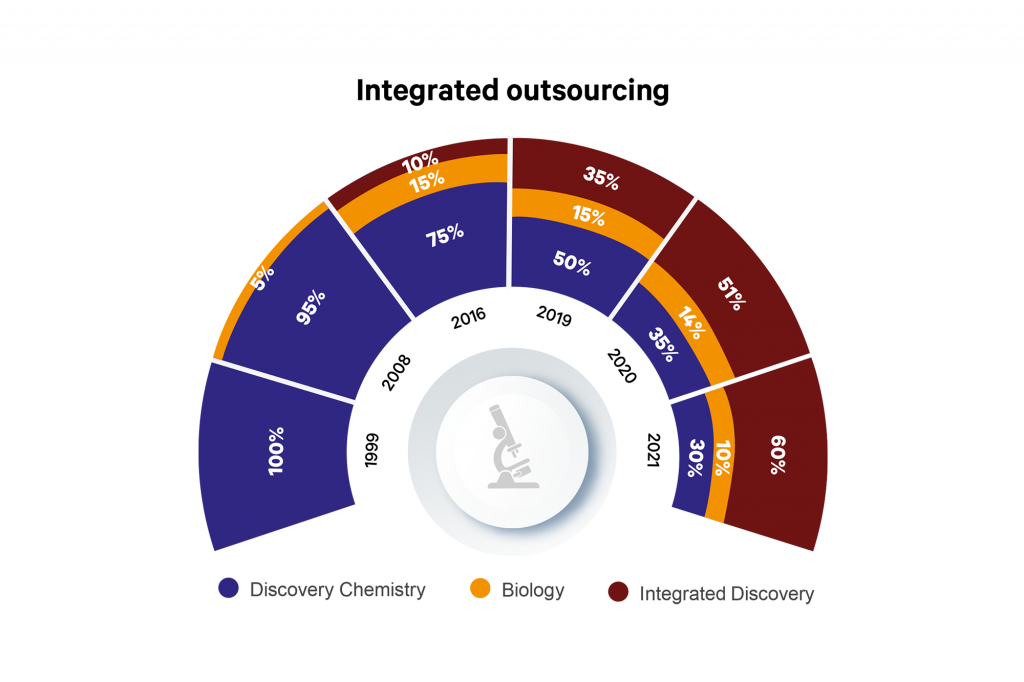
Sai Life Sciences started life as a chemistry provider in 1999. Over 20 years of investment and growth later, Sai Life Sciences has robust CRO and CDMO divisions, with Discovery and CMC capabilities overlapping on a campus in Hyderabad, India, supported by centres of excellence in process chemistry R&D in the UK and exploratory biology in Boston, USA, as well as a manufacturing site in Bidar, India.
Within the Discovery division, we have seen a large shift towards integrated outsourcing, such that in 2021, 60% of customers are working with Sai for more than one Discovery discipline. In addition, we have seen a shift towards programmes moving out of Discovery and into Development at Sai, with outsourcing companies being comfortable with the culture and capability fit of Sai with their organisations.
A CRO/CDMO that puts good communication and high visibility in all aspects of its services has a good opportunity to build trust and long-lasting partnerships with clients. The decade-long relationships with companies and individuals that Sai established is testimony to that culture.
At Sai, we have also seen a shift in the start point of customer outsourcing. As a heritage chemistry company, many of our projects started with chemistry then added on other disciplines such as ADME and toxicology. In-vitro pharmacology was added much later. However, in recent years, an increasing number of companies are outsourcing exploratory biology initially, and moving to add in chemistry when their projects are ready – our exploratory biology team in Boston, which works closely with US-based companies, acts as a funnel to our pipeline of integrated projects in the Hyderabad campus. The CMC division of Sai has also seen a shift in the start point of projects. Historically, manufacturing was a start point for CMC outsourcing, but now CMC externalisation begins during the overlap phase with Discovery.
Conclusion
The contract research and development environment is currently flourishing. Given the external pressures on Pharma to reduce cost, this looks unlikely to change in the near term. Integrated providers of both Discovery and Development are increasing in popularity for ease of contracting, project management, logistics and the cultural fit. Forging a path through Discovery and into Development with the same integrated partner where a good relationship, expertise and capabilities exist is of benefit to smoothing the path of outsourcing in the life sciences industry.