Trends in High Throughput Screening
What is High Throughput Screening?
The virtue of HTS
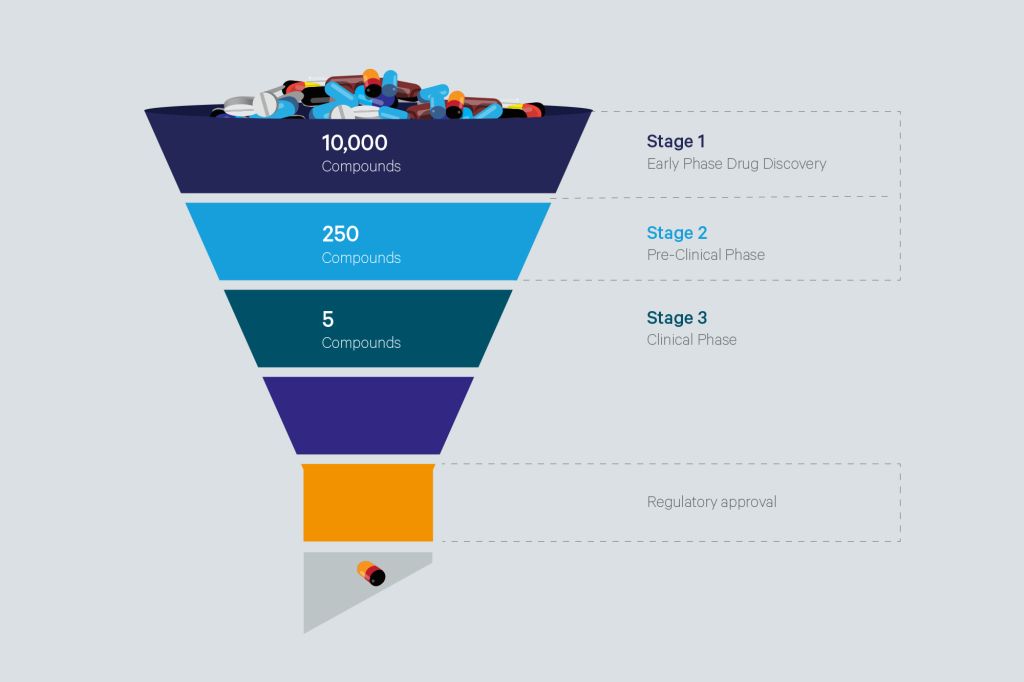
If a methodology has to be nominated to the Science Hall of Fame for its contributions in the field of Biology and Chemistry and its interfaces, this is with no doubt High Throughput Screening (HTS). The concept is simple: testing large libraries of entities -compounds, biologicals - for a selected outcome -e.g. a biological activity, a chemical product or biotechnological end-point.
HTS Story
A Glance Behind the scenes
Popularized at the end of the 1980s by the pioneering drug discovery stories of natural product screenings at Pfizer 1 , the approach has been successfully applied to many fields: in clinical diagnostics (e.g. HPV assay from BD ), in Industrial Biotechnology for microorganism optimization, or catalyst selection in Process chemistry - building its own reputation and name.
Nevertheless, pharmaceutical research- its original application - has the potential to cause the greatest impact for society. HTS has contributed to the development of diverse blockbuster drugs , some of them nowadays belonging to the WHO Essential Medicines List (EML) 2. (See Table 1)
Table 1. Selection of marketed chemical drugs originated from HTS screenings 3,4
| Drug (US trade name; company) | Indication | Target class | Highlights |
| Sitagliptin (Januvia; Merck & Co) | Diabetes (type 2) | Peptidase | Blockbuster |
| Cinacalcet (Sensipar; Amgen) | Hyperparathyroidism | Receptor | Blockbuster |
| Memantine (Namenda; Forest) | CNS | Unknown | Blockbuster |
| Raltegravir (Isentress; Merck & Co.) | HIV Infection | Integrase | Blockbuster |
| Dasatinib (Sprycel; Bristol-Myers Squibb) | Cancer | Tyrosine kinase | WHO essential medicine |
| Bedaquiline (Sirturo; Johnson & Johnson) | Tuberculosis | Proton pump (of mycobacterial ATP synthase) | WHO essential medicine |
| Imatinib (Gleevec; Novartis) | Leukemia | Tyrosine kinase | Blockbuster + WHO essential medicine |
After ca. 30 years of its breakthrough, nowadays HTS is often the best choice to assess the identification of active compounds influencing potentially druggable biological pathways. In comparison to other lead discovery strategies -i.e. fragment screening, structure-based design or virtual screening, HTS offers the following advantages:
- Can be applied either as target-based approach -in vitro, by means of biochemical, biophysical assays with isolated proteins-, or in a more physiologically relevant environment -in vivo, by means of a phenotypic-based approach in cells, tissues or animal models.
- Allows to find modulators of cellular/physiological endpoints where no previous knowledge of the target or ligand is available, (e.g. proteins difficult to characterize via crystallography), or where a target isolation is not suitable (e.g. protein coupled receptors, signalling pathways like induction of particular genes).
- The level of miniaturization, cost-effectiveness and time efficiency possible with the existing technology enable the execution of hundreds of thousands of tests in few days (depending on the target), ultimately achieving the label of uHTS (ultra HTS).
- It is the method of excellence to test biolocal library types such as antibodies, siRNA, miRNA.
HTS is a versatile tool but is not a one-fits-all solution. The available data, the scientific questions and the threshold goals on activity and specificity would determine which drug discovery method is best suited for each specific project.4
Figure 1. Evidencing the great impact of HTS in life sciences.
HTS workflow: the Symphony Orchestra
“Running a HTS is a true symphony piece orchestrated by skilled experts. The success on the day of the concert depends on the previous preparation of all players and the harmonization between them”.
Assay development and validation
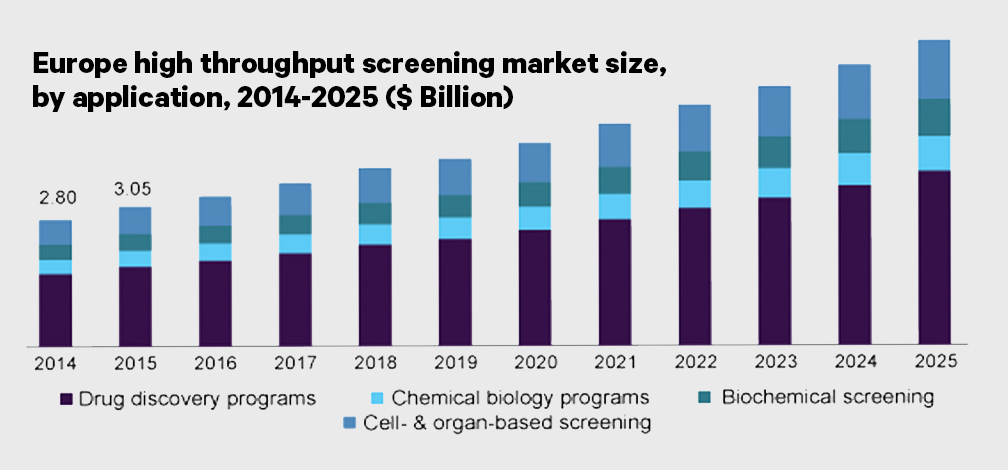
To ensure the success of the screening campaign, each step is crucial. The starting point is the selection of the biological response to monitor and the development of a representative assay that efficiently measures the target, pathway or phenotypic modulation.
HTS implementation
After validation of the assay strategy, the process is upscaled to a primary screening format, by means of implementing it into the modern automation platforms, which integrate robotics, liquid handling devices, highly miniaturized assay vessels like microplates, sensitive detectors as well as control and data processing software.
Notably, keeping the assay flexible and modular allows to adapt to changing needs, e.g. moving from large libraries to smaller focused collections, where more replicates or end-points might be requested.
Primary screening and hit confirmation
Screening of the selected libraries could lead to the identification of hits – a library entity causing the desired biological effect relative to the predefined thresholds of relevance of the screening campaign.
Hits are not yet drugs. Additional medium/high throughput assays might be required to detect false positives or disclaim other assay interferences. Confirmatory tests involve orthogonal assays and the obtention of concentration-response curves. Notably, simultaneous screening and titration-response is possible for some applications by means quantitative HTS (qHTS) designs. Of note, resynthesis of the molecule is often part of hit confirmation.
HTS involvement in the Hit-to-lead process
In the early Drug discovery stages, HTS is also applied to evaluate important properties such ADME and toxicity (for example for CYP inhibition or DILI HTS). Screening technologies allow to speed up the multi-dimensional optimization during hit optimization, which ultimately could result on a lead – a compound which meets the targeted pharmacological and pharmacokinetic properties, but might be still subject of further optimization. The longest process start afterwards, to eventually deliver the final new molecular identity (NME) to the market.
Figure 2. The sheet music of the HTS symphony.
Perspectives in HTS labware
The rapid technological and scientific advances are greatly impacting HTS concept and methodology in many aspects. Based on product and service, the following cutting-edge technologies are worth to be mentioned:
Testing vessel
- The classical assay container is a microplate, which has been miniaturized from the original 96-well to the currently most common 1536-well format. Although even 9600-well plates are available, its applicability is limited by the assay readout system.
- “Plate-free” formats have been mainly used for academic purproses. With the nanobiotechnology outbreak, microchip-based systems like chemical microarrays for HTS were popularized.
Figure 3. HTs robot for small format of microplates, microplate handling

Liquid handling
- Test item management is nowadays fully robotized. Typically, test items are placed in stock plates as (DMSO) solution.
- Nanoliter dispensing allows a highly precise and contact-free transfer of a variety of liquids, avoiding cross-contamination. The acoustic droplet dispensing (ADE) technology is specially well suited for the transfer of stock solutions, for example, the Echo650 ADE from Beckman Coulter can eject tiny droplets from 2.5 nl.
- Other reagent dispenser types like Multidrop might be more appropiate during other assay preparation and workout steps.
Automation, robotics and software
- Nowadays, HTS labs should be able to screen large libraries in a variety of test systems and assay formats. The level of speed, accuracy and flexibility has been achieved thanks to the integrated modular lab automation systems
- The introduction of artificial intelligence (AI) and advanced robotics enables a closer interaction during the screening and might speed the resolution of errors.
- Thanks to software advances, automated quality control is also possible, allowing for example real-time monitoring of liquid dispensers. Moreover, it makes possible to fix remotely any issue encountered in the process.
- Open source automation is a reality. Great efforts from the academic community has lead to open source microscopy tools and extensible hardware platforms such as 3D printers.
- Software for data analysis has evoluted with the HTS systems, allowing the processing of the massive amount of data acquired in the screening campaigns with a high level of quality.
Figure 4. integrated modular lab automation systems example.

According to AstraZeneca reasearchers, “Modular automation minimises equipment redundancy and provides the ability to multiplex different assays on the same screening platform, bringing further efficiencies”.5
Readout system
The biological question determines which readout can be used: chemical, biophysical, biochemical or cell-based.
- Chemical analysis is extensively used for the evaluation of diverse DMPK properties where the compounds per se are monitored. In the last years, the sensitivity and versatility of the UPLC-MS/MS has gone beyond expectations, with systems like Echo® MS for Acoustic Ejection Mass Spectrometry (AEMS) or Triple Quad™ 7500 LC-MS/MS (QTRAP® Ready ). Allowing very small lower limits of quantitation (LLOQs) or high-throughput metabolite analysis for metabolomic applications.
- For biological readouts, the development of multiplexed plate readers and real-time analysers like live cell imaging systems has greatly impacted the primary screen concept.
- The use of biophysical methods is led by SPR . Lately, other technologies have been commercially implemented for higher throughput: Microscale thermophoresis (MST) and Nanoscale differential scanning fluorimetry (nanoDSF).
- Classical biochemical direct fluorescence/luminescent assays have been upgraded to configurations that reduce assay interferences, and/or allow the monitoring of membrane coupled receptors or protein-protein interactions by means of proximity-based approaches (FRET, BRET, HTRF, AlphaSreen, LanthaScreen ).
- High content screenings (HCS) are a type of phenotypic screening in which multiple parameters can be recorded over time using automated imaging (e.g. morphology, viability, spatial distribution of tagged proteins). To this end, powerful automated microscope systems and imaging analysis are available on the market. They make it possible to monitor a variety of living systems, from single-cells to living organisms such as zebrafish.
- High Throughput Flow Cytometry (HTFC™), a phenotypic screening type based on the multiplexed fluorescence intensity design, is specially well suited for screening suspension cells where multiple readouts are desired.
- For HTS to search gene expression modulators, (luciferase) reporter gene assays have lead the field for more than a decade. Given the high rates of false hits , its limited resemblance to natural expression context (as mRNA is expressed from a plasmid-based construct), and impossibility to be used on non-immortalized cells, alternatives are being introduced. High-throughput RT-qPCR is presented as an alternative overcoming these issues. The introduction of 1,536-well qPCR labware by Roche has already allowed high-throughput commerical applications in the diagnostic field.
Advances in HTS test systems
Revolution on the test systems and targeted biological responses in HTS has been possible thanks to two main factors: evolution of the readout systems and advanced data analysers, and the explosion of the “Omics research” – genomics, proteomics and transcriptomics - allowing the engineering and culturing of more physiologically-relevant in vivo models. The following mainstream innovations are invading the HTS market:
- CRISPR-Cas9 technology is being used as power tool to engineer more-physiologically relevant cellular screening assays.
- The efforst invested in 3D culture systems were worth it, 3D spheroids have been commercialized and applied in HTS.
- 4D assays add the dimension of time into the experiment, which is specially interesting for the study of 3D tumor models. Although not applied in big screenings yet, the approach has been useful for later stages of hit qualification.
- Initial efforts to obtain organoids were based on the use of cell-derived cultures, but bioprinting technology is gaining position in the field.
- As a next level of complexity, tissue models and organ-on-chip devices have been developed. Those methods areworth being used in lead validation phases. For example, multiorgan-on-a-Chip models have been designed to study ADME properties and get a better estimate of the human pharmacokinetic of clinical candidates.
Conclusions
Who plays next?
The field of HTS is gigantic and under continous expansion. Like in a symphony, many players are involved. An advance in a single player (like a new target, automation system or engineered test model) will unlikely result in a drastic change of the melody. An screening with a novel “human-on-a-chip” model will not be possible unless the robotic systems, data analysers and readout technologies make a step forward too.
Bibliography
- Pereira, D. A. & Williams, J. A. Origin and evolution of high throughput screening. Br. J. Pharmacol. 152, 53 (2007).
- World Health Organization. World Health Organization model list of essential medicines: 21st list 2019. https://apps.who.int/iris/handle/10665/325771.
- Swinney, D. C. & Anthony, J. How were new medicines discovered? Nat. Rev. Drug Discov. 10, 507–519 (2011).
- Macarron, R. et al. Impact of high-throughput screening in biomedical research. Nat. Rev. Drug Discov. 10, 188–195 (2011).
- Plant, D. et al. Laboratory automation in early drug discovery. Drug Target Review vol. 7 21–24 (2020).